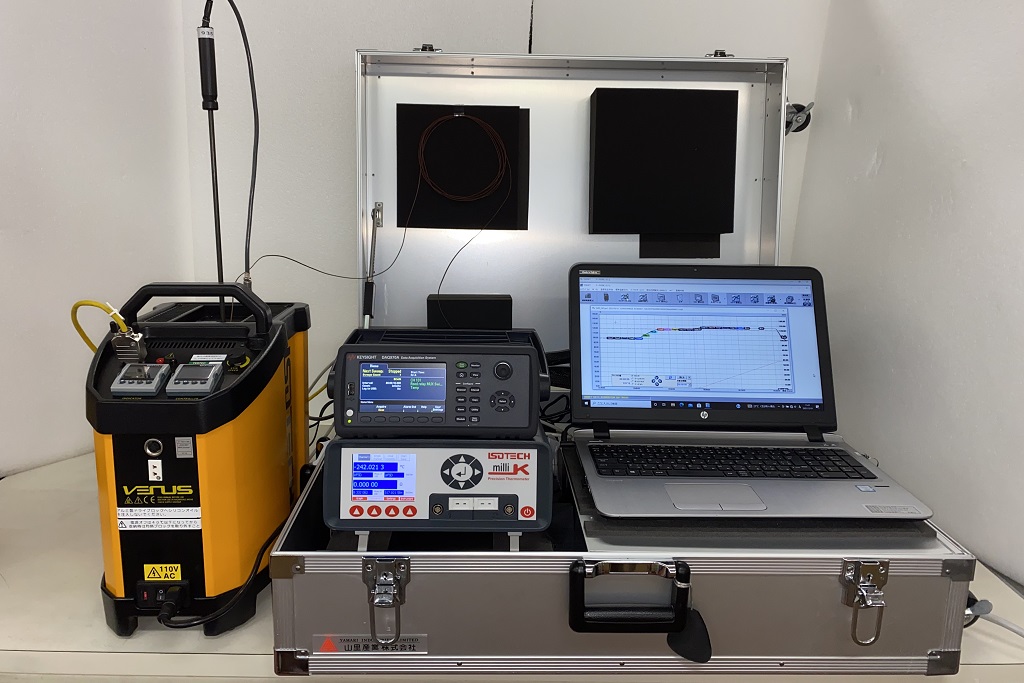
Field validation measurement service
Sterilization validation such as autoclaving and tunnel drying
The sterilization procedure is carried out in final containers or packages, and the sterilization of microorganisms after sterilization is quantitatively measured to reduce the number of microorganisms to 1/1 million or less of the sterility assurance level. For example, we measure F0 with a high degree of accuracy and provide support from the actual operation to the preparation of a report based on a sentence stating that it was a sterilization method capable of estimating SAL≦10-6.